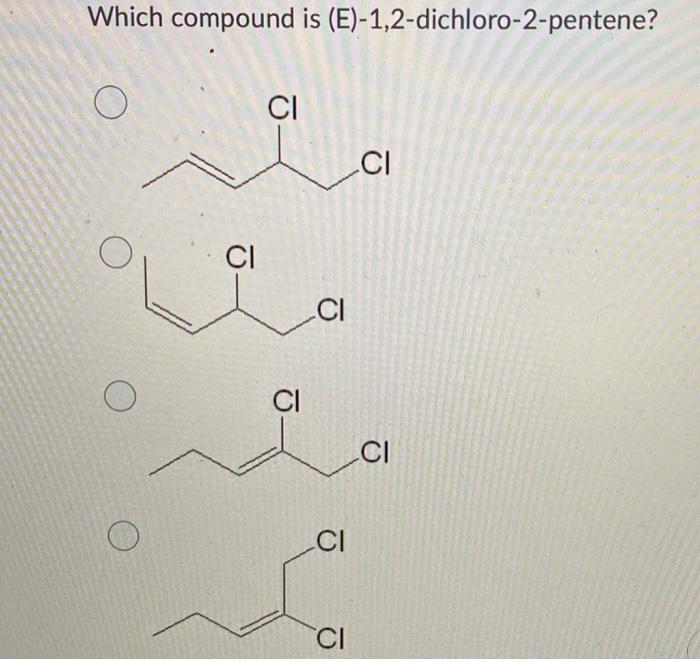
E 1 2 dichloro 2 pentene – Prepare to delve into the captivating world of 1,2-dichloro-2-pentene. As we embark on this journey, we will uncover its molecular structure, synthesis methods, diverse applications, safety considerations, reactivity, spectroscopic analysis, and environmental impact.
From its industrial significance to its potential risks, this comprehensive guide will shed light on every aspect of 1,2-dichloro-2-pentene, providing a thorough understanding of this versatile chemical compound.
Chemical Structure and Properties
- ,2-dichloro-2-pentene is an organic compound with the molecular formula C5H8Cl2. It is a structural isomer of 1,1-dichloro-2-pentene. 1,2-dichloro-2-pentene is a colorless liquid with a sharp, pungent odor. It is slightly soluble in water and more soluble in organic solvents.
- ,2-dichloro-2-pentene is a reactive compound. It can undergo a variety of reactions, including addition, substitution, and elimination reactions. It is also a flammable compound.
Physical Properties, E 1 2 dichloro 2 pentene
- Colorless liquid
- Sharp, pungent odor
- Slightly soluble in water
- More soluble in organic solvents
- 70 °C
Density
1.15 g/mL
Boiling point
160-162 °C
Melting point
Chemical Properties
- Reactive compound
- Can undergo addition, substitution, and elimination reactions
- Flammable compound
Synthesis and Production
,2-dichloro-2-pentene can be synthesized through various methods, including:
- Dehydrochlorination of 1,1,2-trichloro-2-pentene:This involves removing one molecule of hydrogen chloride (HCl) from 1,1,2-trichloro-2-pentene, resulting in the formation of 1,2-dichloro-2-pentene.
- Addition of chlorine to 2-pentene:This reaction entails adding chlorine gas (Cl2) to 2-pentene in the presence of a radical initiator, leading to the formation of 1,2-dichloro-2-pentene.
- Hydrochlorination of 2-pentyne:This process involves adding hydrogen chloride (HCl) to 2-pentyne in the presence of a Lewis acid catalyst, resulting in the formation of 1,2-dichloro-2-pentene.
Industrially, 1,2-dichloro-2-pentene is produced via the dehydrochlorination of 1,1,2-trichloro-2-pentene. This process is carried out in the presence of a base catalyst, such as sodium hydroxide (NaOH), at elevated temperatures. The reaction proceeds through an E2 elimination mechanism, resulting in the removal of HCl and the formation of 1,2-dichloro-2-pentene.
Applications and Uses
,2-dichloro-2-pentene finds applications in various fields, including chemical synthesis, industry, and other specialized areas. Its unique chemical properties make it a versatile compound with diverse uses.
Chemical Synthesis
In chemical synthesis, 1,2-dichloro-2-pentene serves as a valuable intermediate in the production of various organic compounds. It undergoes reactions such as addition, substitution, and elimination to yield a wide range of products. For instance, it can be used to synthesize pharmaceuticals, fragrances, and flavors.
Industrial Applications
,2-dichloro-2-pentene has industrial applications in the manufacturing of plastics, solvents, and coatings. It is used as a comonomer in the production of polyvinyl chloride (PVC), a widely used plastic material. Additionally, it is employed as a solvent in the electronics industry and as a component in paint formulations.
Other Applications
Beyond chemical synthesis and industrial uses, 1,2-dichloro-2-pentene has specialized applications in other fields. It is utilized in the production of certain types of adhesives, sealants, and lubricants. Furthermore, it finds use in the synthesis of agrochemicals and as an intermediate in the manufacture of fragrances.
Safety and Handling
,2-dichloro-2-pentene is a highly flammable liquid with a strong odor. It is also corrosive and can cause skin and eye irritation. When handling this chemical, it is important to take the following precautions:* Wear appropriate personal protective equipment, including gloves, safety glasses, and a respirator.
E 1 2 dichloro 2 pentene, an organic compound with a unique chemical structure, has a wide range of applications in various industries. Its versatility is akin to the bustling flower industry, where a flower store sold 2450 blooms in a single day.
Just as flowers bring joy to countless individuals, e 1 2 dichloro 2 pentene plays a crucial role in enhancing our daily lives.
- Handle the chemical in a well-ventilated area.
- Keep the chemical away from heat, sparks, and open flames.
- Do not smoke or eat while handling the chemical.
- Wash your hands thoroughly after handling the chemical.
Emergency Procedures
In case of a spill, leak, or fire, follow these emergency procedures:* Evacuate the area immediately.
- Call 911 or your local emergency number.
- Contain the spill or leak if possible.
- Do not attempt to clean up the spill or leak yourself.
- Stay away from the area until emergency responders arrive.
Reactivity and Reactions
,2-dichloro-2-pentene is a highly reactive compound due to the presence of two chlorine atoms attached to the same carbon atom. These chlorine atoms make the carbon-carbon double bond more susceptible to electrophilic attack.
Addition Reactions
,2-dichloro-2-pentene undergoes addition reactions with a variety of electrophiles. For example, it reacts with hydrogen bromide to form 1,2-dibromo-2-pentene. The reaction proceeds via an electrophilic addition mechanism, in which the hydrogen bromide molecule adds to the double bond, with the hydrogen atom adding to the carbon atom bearing the two chlorine atoms and the bromine atom adding to the other carbon atom.
Spectroscopy and Analysis: E 1 2 Dichloro 2 Pentene
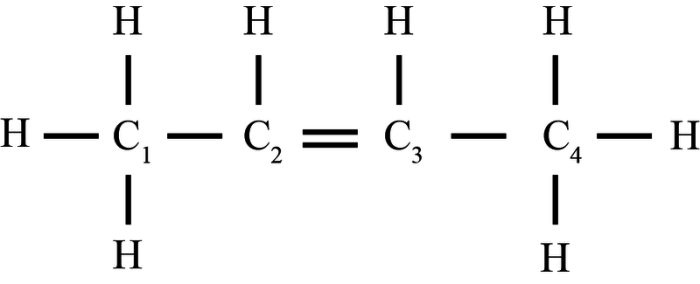
1,2-Dichloro-2-pentene can be analyzed using various spectroscopic techniques. These techniques provide valuable information about the molecular structure, functional groups, and chemical composition of the compound.
Infrared Spectroscopy
- Infrared (IR) spectroscopy measures the absorption of infrared radiation by the sample. Different functional groups absorb IR radiation at specific frequencies, providing information about their presence and identity.
- For 1,2-dichloro-2-pentene, the characteristic IR absorption bands include:
- C-H stretching: 2960-2860 cm -1
- C=C stretching: 1640-1620 cm -1
- C-Cl stretching: 760-680 cm -1
Nuclear Magnetic Resonance Spectroscopy
- Nuclear Magnetic Resonance (NMR) spectroscopy provides information about the types and connectivity of atoms within a molecule.
- For 1,2-dichloro-2-pentene, the 1H NMR spectrum shows:
- A triplet at 5.6 ppm (1H) for the proton on the carbon adjacent to the double bond
- A quartet at 2.3 ppm (2H) for the protons on the carbon adjacent to the chlorine atoms
- A singlet at 1.8 ppm (3H) for the methyl protons
- The 13C NMR spectrum shows:
- A peak at 127 ppm for the carbon of the double bond
- A peak at 45 ppm for the carbon adjacent to the chlorine atoms
- A peak at 25 ppm for the carbon adjacent to the double bond
- A peak at 15 ppm for the methyl carbon
Mass Spectrometry
- Mass spectrometry (MS) measures the mass-to-charge ratio of ions produced from the sample.
- The mass spectrum of 1,2-dichloro-2-pentene shows a molecular ion peak at m/z = 152, corresponding to the molecular formula C 5H 8Cl 2.
- Other significant peaks include:
- m/z = 117 (loss of a chlorine atom)
- m/z = 89 (loss of two chlorine atoms)
- m/z = 55 (loss of the pentene fragment)
Environmental Impact and Toxicity
,2-dichloro-2-pentene has the potential to cause adverse effects on the environment and human health. Its environmental impact is primarily due to its volatile nature and potential for contamination of air, water, and soil. The toxicity of 1,2-dichloro-2-pentene is primarily attributed to its reactive chlorine atoms, which can interact with biological molecules and cause damage to cells and tissues.
Environmental Impact
The release of 1,2-dichloro-2-pentene into the environment can have several negative consequences. Its volatility allows it to readily evaporate and disperse into the atmosphere, where it can contribute to the formation of ground-level ozone and smog. These air pollutants can cause respiratory problems, eye irritation, and damage to plants and ecosystems.
Additionally, 1,2-dichloro-2-pentene can contaminate water sources through runoff or spills, posing a threat to aquatic life and potentially affecting human health through the consumption of contaminated water.
Toxicity
Exposure to 1,2-dichloro-2-pentene can cause a range of adverse health effects. Inhalation of its vapors can irritate the respiratory tract, leading to coughing, wheezing, and difficulty breathing. Contact with skin or eyes can cause irritation, redness, and pain. Prolonged or high-level exposure to 1,2-dichloro-2-pentene can damage the liver, kidneys, and central nervous system.
The International Agency for Research on Cancer (IARC) has classified 1,2-dichloro-2-pentene as a possible human carcinogen, based on limited evidence from animal studies.
Query Resolution
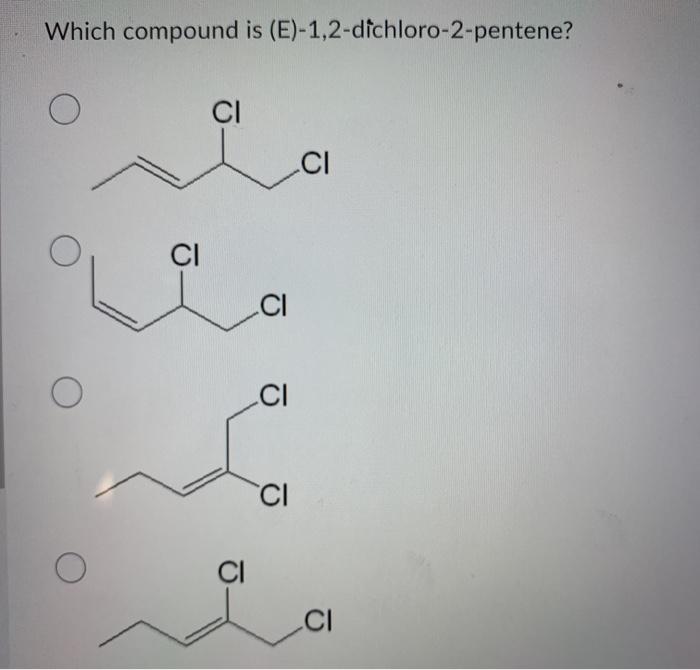
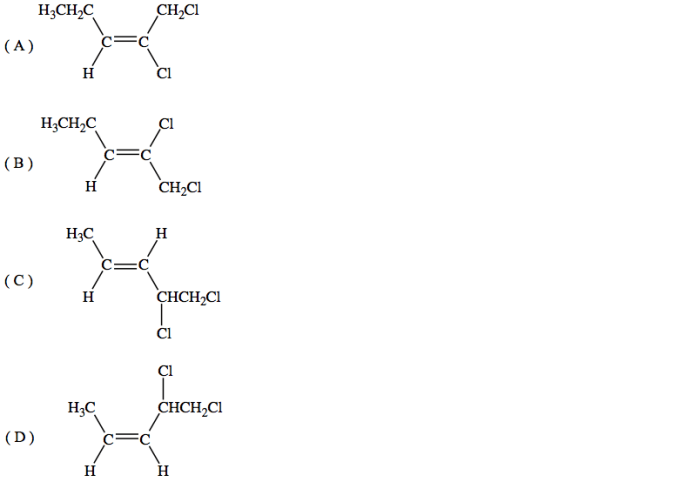
What is the molecular structure of 1,2-dichloro-2-pentene?
1,2-Dichloro-2-pentene is an organic compound with the molecular formula C5H8Cl2. It is a branched hydrocarbon with a double bond between the second and third carbon atoms and two chlorine atoms attached to the second carbon atom.
How is 1,2-dichloro-2-pentene synthesized?
1,2-Dichloro-2-pentene can be synthesized through various methods, including the hydrochlorination of 1-pentene, the dehydrochlorination of 1,2,2-trichloropentane, and the Diels-Alder reaction of 1,3-butadiene with dichloroketene.
What are the applications of 1,2-dichloro-2-pentene?
1,2-Dichloro-2-pentene is primarily used as an intermediate in the production of other chemicals, such as vinyl chloride and chloroprene. It is also used as a solvent and in the synthesis of pharmaceuticals, fragrances, and flavors.