Endothermic reaction vs exothermic reaction worksheet answer key – Delve into the captivating realm of endothermic and exothermic reactions with our comprehensive worksheet answer key. This in-depth guide unravels the intricate energy changes that define these reactions, empowering you to grasp their fundamental principles and practical applications.
Embark on a journey through the energetic landscape of chemical reactions, where endothermic processes absorb energy from their surroundings, while exothermic reactions release energy into the environment. Explore the factors that govern their rates and delve into the diverse applications that make these reactions indispensable in our daily lives.
Endothermic vs Exothermic Reactions
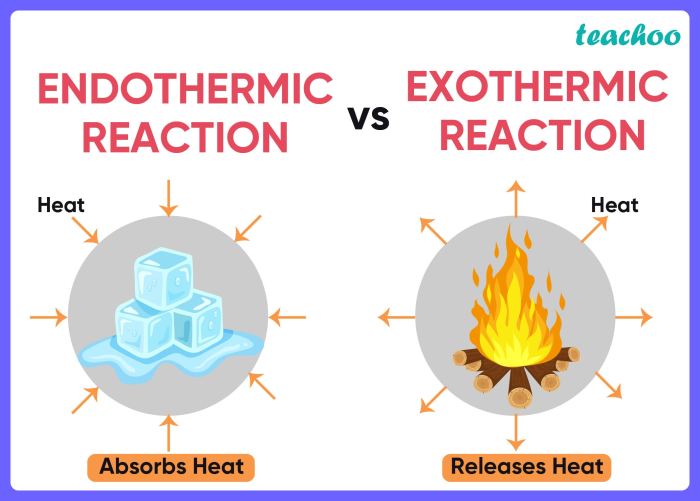
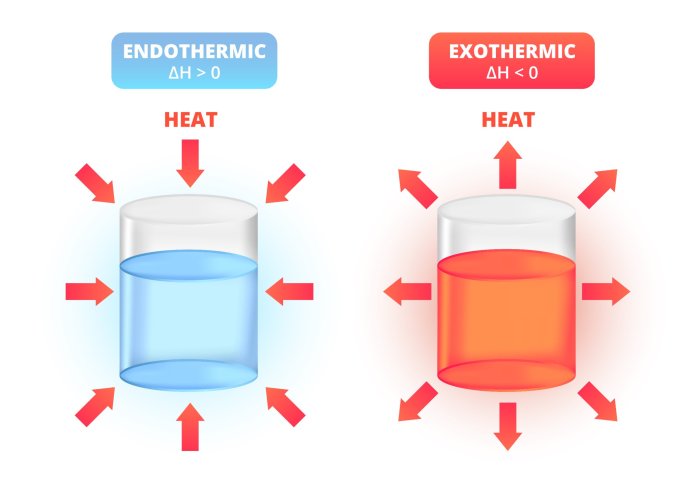
Endothermic and exothermic reactions are two types of chemical reactions that differ in the way they exchange energy with their surroundings.
Endothermic Reactions
- Endothermic reactions absorb energy from their surroundings.
- The products of an endothermic reaction have more energy than the reactants.
- Endothermic reactions typically require an input of energy, such as heat or light, to proceed.
Exothermic Reactions, Endothermic reaction vs exothermic reaction worksheet answer key
- Exothermic reactions release energy into their surroundings.
- The products of an exothermic reaction have less energy than the reactants.
- Exothermic reactions typically proceed spontaneously, releasing heat or light as a byproduct.
Energy Changes in Reactions
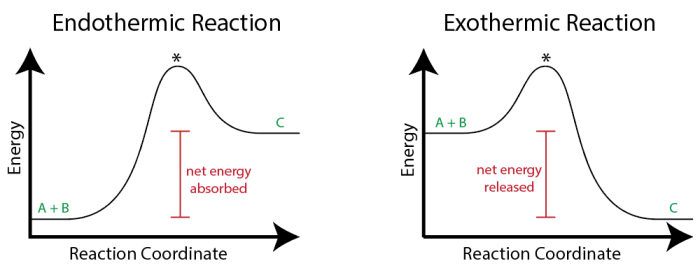
In endothermic reactions, the energy of the products is greater than the energy of the reactants. This means that the reaction requires an input of energy to proceed. The energy is typically supplied in the form of heat or light.
In exothermic reactions, the energy of the products is less than the energy of the reactants. This means that the reaction releases energy into the surroundings. The energy is typically released in the form of heat or light.
The following diagram illustrates the energy changes in endothermic and exothermic reactions:
- Endothermic reaction: Reactants (lower energy) → Products (higher energy) + Energy (absorbed)
- Exothermic reaction: Reactants (higher energy) → Products (lower energy) + Energy (released)
Factors Affecting Reaction Rates
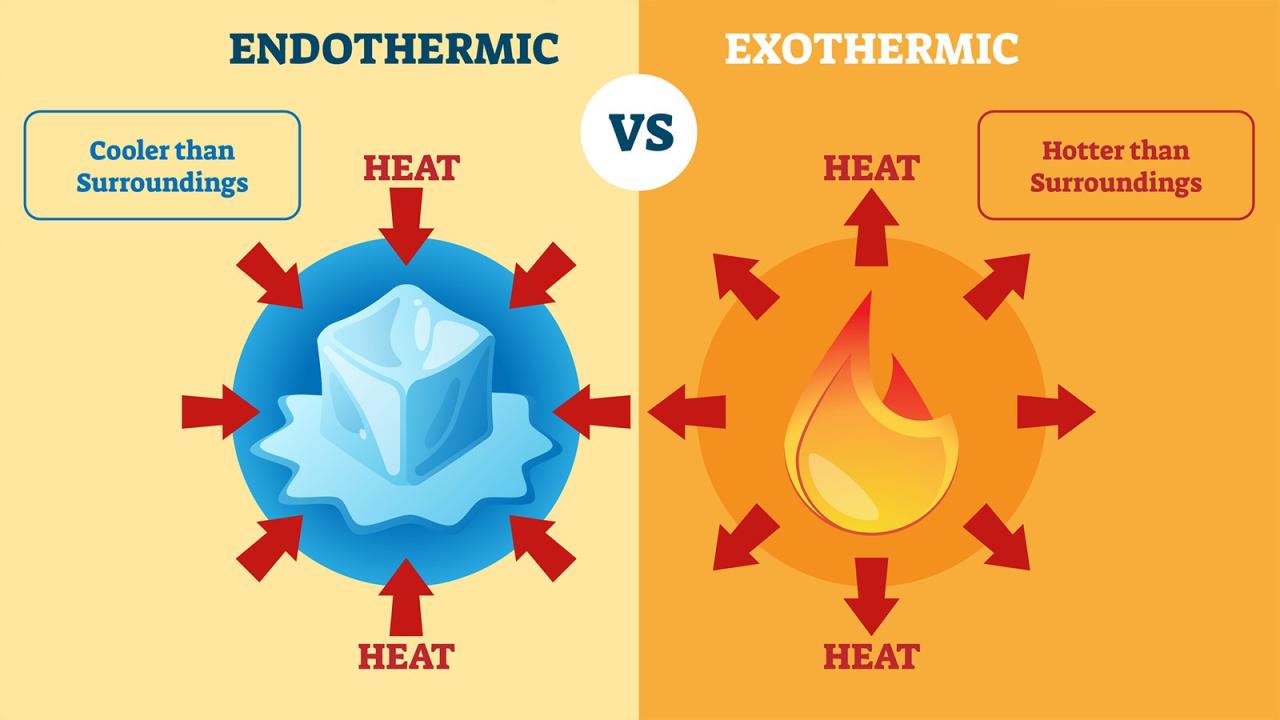
The rates of endothermic and exothermic reactions are affected by several factors, including:
| Factor | Effect on Endothermic Reactions | Effect on Exothermic Reactions |
|---|---|---|
| Temperature | Increased temperature increases the rate of endothermic reactions. | Increased temperature decreases the rate of exothermic reactions. |
| Concentration of reactants | Increased concentration of reactants increases the rate of both endothermic and exothermic reactions. | Increased concentration of reactants increases the rate of both endothermic and exothermic reactions. |
| Surface area of reactants | Increased surface area of reactants increases the rate of both endothermic and exothermic reactions. | Increased surface area of reactants increases the rate of both endothermic and exothermic reactions. |
| Presence of a catalyst | A catalyst can increase the rate of both endothermic and exothermic reactions. | A catalyst can increase the rate of both endothermic and exothermic reactions. |
Applications of Endothermic and Exothermic Reactions
Endothermic and exothermic reactions are used in a variety of applications, including:
Endothermic Reactions
- Cooling systems (e.g., air conditioners, refrigerators)
- Chemical manufacturing (e.g., production of fertilizers, plastics)
- Food processing (e.g., freezing, drying)
Exothermic Reactions, Endothermic reaction vs exothermic reaction worksheet answer key
- Heating systems (e.g., furnaces, boilers)
- Power generation (e.g., combustion engines, nuclear reactors)
- Welding and cutting
Worksheet Answer Key
-*Question 1
Which of the following is an example of an endothermic reaction?
-*Answer
Melting of iceQuestion 2: Which of the following is an example of an exothermic reaction?
-*Answer
Burning of woodQuestion 3: What is the difference between an endothermic and an exothermic reaction?
-*Answer
Endothermic reactions absorb energy from their surroundings, while exothermic reactions release energy into their surroundings.Question 4: What factors affect the rates of endothermic and exothermic reactions?
-*Answer
Temperature, concentration of reactants, surface area of reactants, and presence of a catalyst.Question 5: Give an example of an application of an endothermic reaction.
-*Answer
Air conditioningQuestion 6: Give an example of an application of an exothermic reaction.
-*Answer
Heating a house with a furnace
Questions Often Asked: Endothermic Reaction Vs Exothermic Reaction Worksheet Answer Key
What is the key difference between endothermic and exothermic reactions?
Endothermic reactions absorb energy from their surroundings, while exothermic reactions release energy into the environment.
How can I identify an endothermic reaction from an exothermic reaction?
Endothermic reactions typically require an external energy source, such as heat, while exothermic reactions release heat or light.
What factors influence the rate of an endothermic or exothermic reaction?
Temperature, concentration, surface area, and the presence of a catalyst can all affect the reaction rate.